Saturation Thermodynamics - Thermodynamics
Thermodynamics Directory | Heat Transfer Directory
Saturation Thermodynamic
The term saturation defines a condition in which mixture of vapor and liquid can exist together at a given temperature and pressure. The temperature at which vaporization (boiling) starts to occur for a given pressure is called the saturation temperature or boiling point. The pressure at which vaporization (boiling) starts to occur for a given temperature is called the saturation pressure.
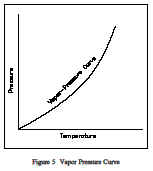
For water at 212F, the saturation pressure is 14.7 psia and, for water at 14.7 psia, the saturation temperature is 212F. For a pure substance there is a definite relationship between saturation pressure and saturation temperature. The higher the pressure,the higher the saturation temperature. The graphical representation of this relationship between temperature and pressure at saturated conditions is called the vapor pressure curve. Atypical vapor pressure curve is shown in Figure 5. The vapor/liquid mixture is at saturation when the conditions of pressure and temperature fall on the curve.