Related Resources: physics
Air Density Equation
Air Density Equation and proof.
Physical properties of air can be represented by the real gas equation, which is the modified version of ideal gas equation.
Dry air obeys the ideal gas law,
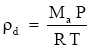
where:
ρd = density of dry air kg - m-3
Ma = molecular weight of air (28.97 kg / mol)
P = barometric pressure (Pa)
R = universal gas constant 8.31432 × 103 N·m·kmol-1· K-1
T = air temperature (K).
At standard temperature (T = T0 = 273.15 K) and pressure (P = P0 = 1013.25 mb), gives ρd0 = 1.2922 kg m-3.
Therefore, our equation also gives:
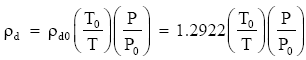
where ρd is in kg m-3 when P is in millibars and T is in kelvins.
Related: